
Conventional Pathways for Hydrogen Production
To meet climate goals, a global transition towards low carbon pathways strongly propagates the idea to shift to green hydrogen.
But the dominance of fossil-based grey hydrogen remains a reality to be acknowledged and addressed. Despite efforts to transition from fossil-based hydrogen, it is expected that grey hydrogen will play a critical role in meeting rising cross-sectoral demand until the green hydrogen value chain matures to commercialisation.
Steam Methane Reforming (SMR) and Coal Gasification are currently the two key processes contributing to most of the hydrogen produced globally. Figure 1 can be referred to understand production volumes for hydrogen globally.

Steam Methane Reforming (SMR) from natural gas or hydrocarbons is the cheapest method to produce industrial hydrogen. This process accounts for 48% of the global hydrogen production. The efficiency of the method is around 70-80% with a Technology Readiness Level (TRL) of 9. The SMR process takes place in two steps. In the first step, natural gas — which contains hydrocarbons such as methane — undergo a thermochemical reaction in the presence of a catalyst (usually nickel) using high temperature steam (700-1000 ºC under 14 - 20 atmospheres of pressure) to produce hydrogen, carbon monoxide (CO) and carbon dioxide (CO2). Then, a water-gas shift reaction takes place where CO and steam are reacted to produce CO2 and additional hydrogen.
In the second step, CO2 and other impurities such as sulphur (S), chlorine (Cl) and carbon oxides, are removed from the gas stream through pressure swing adsorption process to produce 99.99% pure hydrogen.
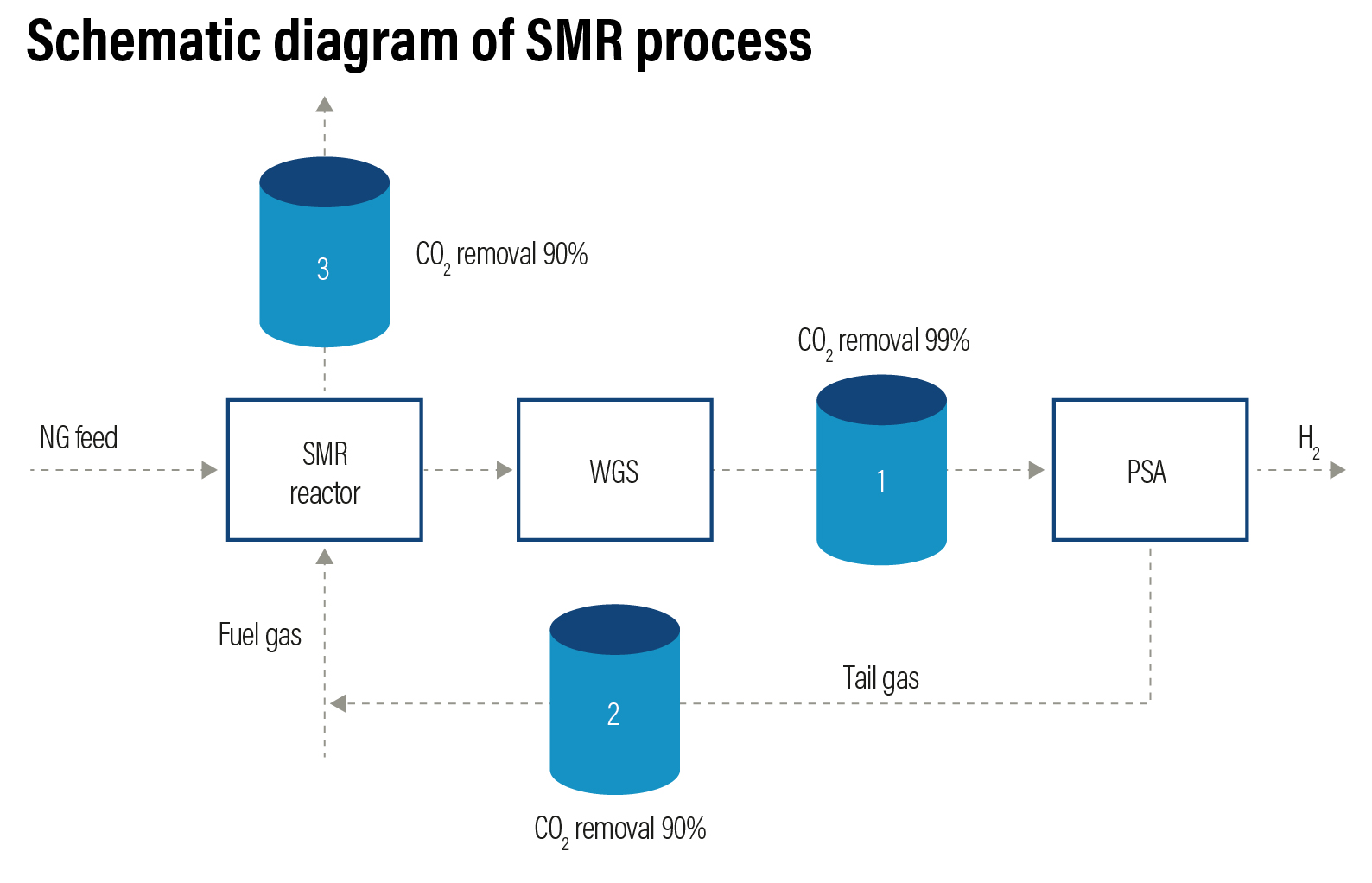
Steam reforming can also produce hydrogen from other fuels such as ethanol, propane, and gasoline. Hydrogen produced from steam methane reformation is coded as grey hydrogen. The challenges associated with SMR is the energy intensiveness of the procedure and the ratio of CO2 emissions to hydrogen produced. Production of 1 kilogram of hydrogen releases anywhere between 9-12 kilogram of CO2, a greenhouse gas that can be captured.
Coal Gasification: Coal gasification accounts for 18% of the global hydrogen production. In this process, carbon-based feedstock or coal is converted into syngas — a mixture of CO, hydrogen, steam, and oxygen — in a gasifier in the presence of steam and oxygen at a very high temperature and moderate pressure. Depending on the gasification technology used, some quantities of water, CO2 and methane can be produced along with the syngas. To produce hydrogen, syngas is moved to a water-gas shift reactor, where CO in the gas is reacted with water to produce additional hydrogen and CO2. These are then separated, producing two hydrogen molecules and three molecules of carbon.
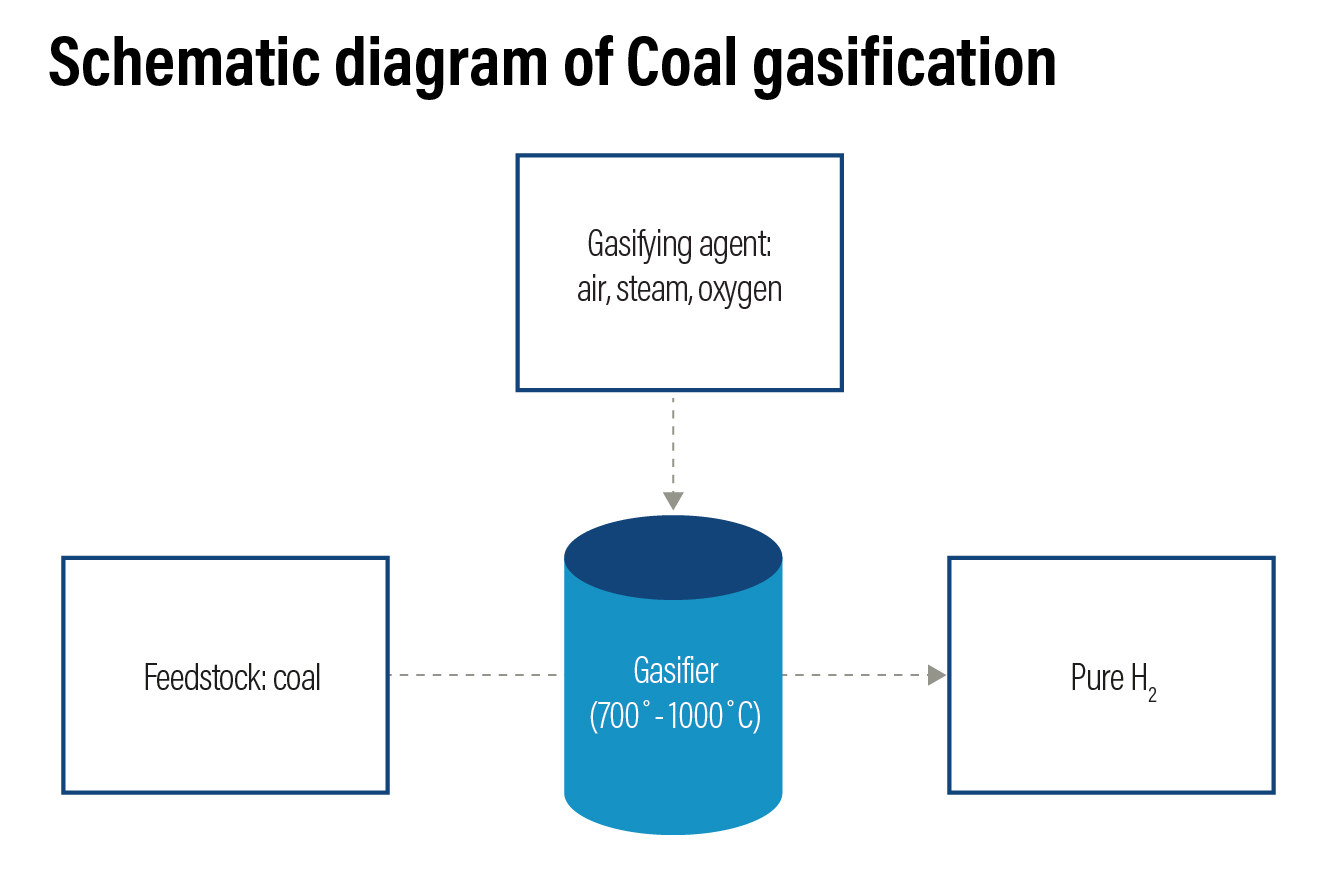
Hydrogen from coal gasification is colour coded as brown hydrogen. The efficiency of the technology is around 60-70% with a technology readiness scale of 8 and has a higher carbon footprint. For every kilogram of hydrogen produced in this method, about 18-20 kilogram of CO2 is released.
Methane Pyrolysis involves thermal decomposition of methane from natural gas. This is an emerging technology to produce hydrogen with lower carbon intensity in comparison to SMR. In this process, methane is thermally split into gaseous hydrogen and solid carbon. New methane is passed through a liquid bubble column at a temperature range of 1,100 to 1,200 ºC without a catalyst. The temperature can be brought down using a catalyst. Molten metals (Ti-Titanium, Pb-Lead, Sn-Tin), molten metal alloys (Ni-Bi Nickel-Bismuth, Cu-Bi Copper-Bismuth) and molten salts (KBr Potassium Bromide, NaBr Sodium Bromide, NaCl Sodium Chloride, NaF Sodium Fluoride, MnCl2 Manganese Chloride, KCl Potassium Chloride) in liquid bubble column reactors are being used as catalysts in recent years. Iron catalyst are most considered stable for methane pyrolysis at temperature of 700-1000 degrees Celsius.
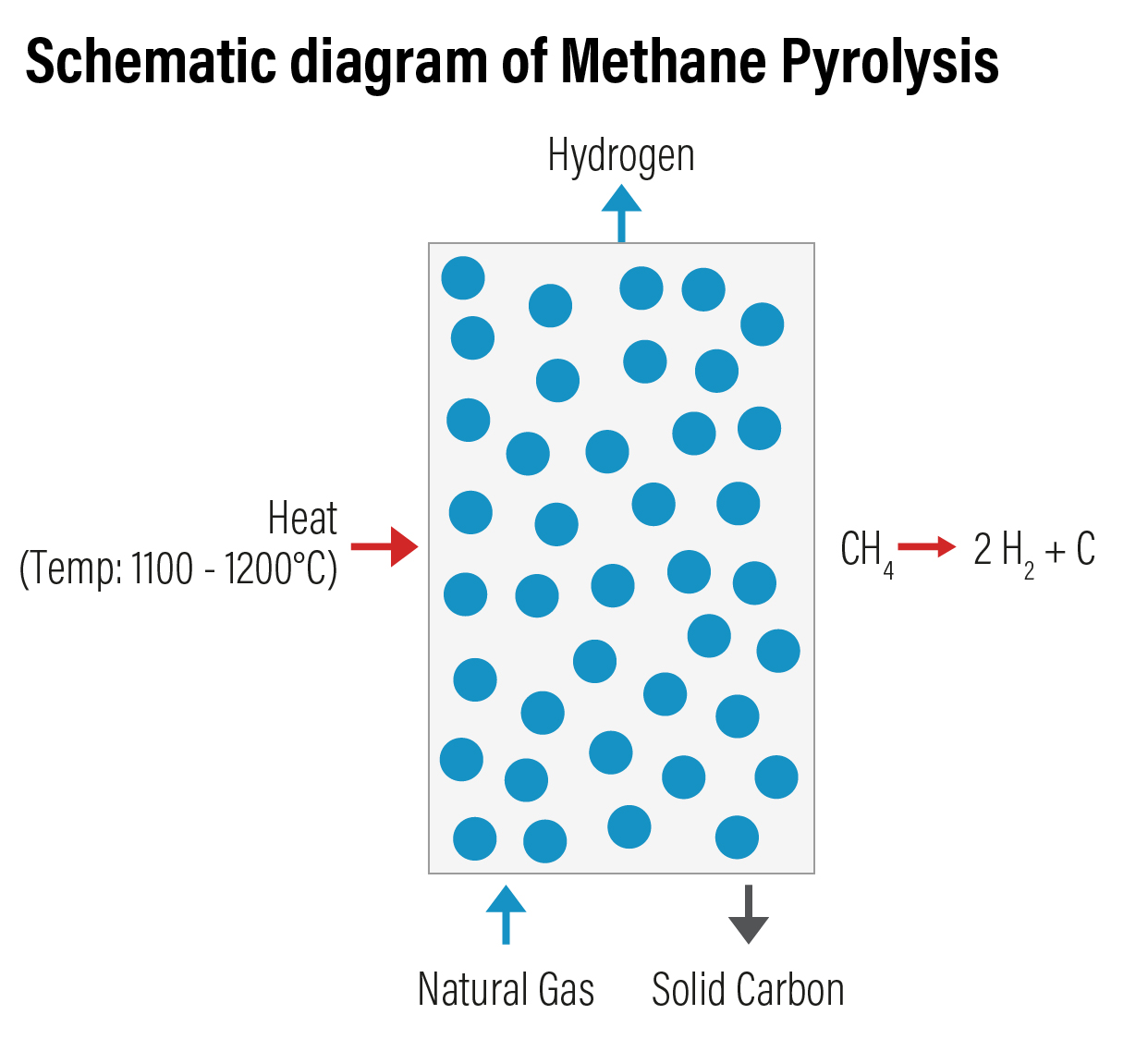
Various reactors types used for the catalytic decomposition of methane are packed-bed reactors, fluidized - bed reactors, fluid – wall reactors, molten-salt reactors, etc. Of these, fluidized -bed reactor is the most used one for methane pyrolysis. Hydrogen produced through this method is coded as turquoise hydrogen.
Unlike other methods that use fossil fuel as feedstock, the advantage with this method is the production of carbon dioxide free hydrogen. Methane pyrolysis has an efficiency of 58%. The solid carbon produced as by-product can be sequestrated permanently. However, the challenge with the process is the formation of soot which leads to deterioration of the operational efficiency and the deactivation of the catalyst surface after a short time.
The following is a comparison of the different processes used for production of Hydrogen using fossil fuels.
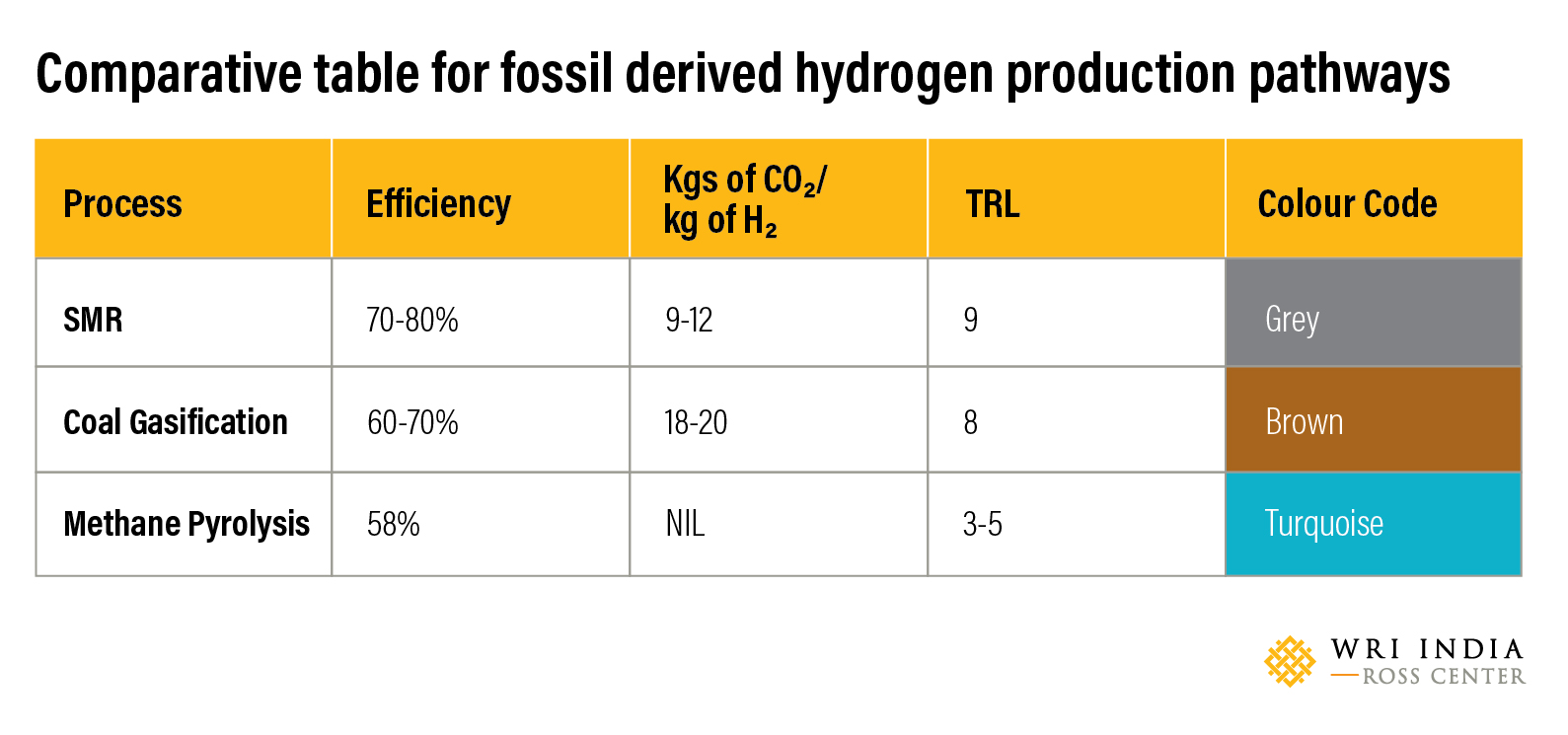
Currently, most of the hydrogen produced in India is through the SMR process. Of late there has been a growing interest in the production of the hydrogen using electrolysis method which have been discussed in earlier blogs. However, it is expected that hydrogen production using fossil fuels will continue to occupy a significant share soon due to the process readiness and efficiencies. Low-carbon production alternatives like methane pyrolysis and SMR and coal gasification with technologies like Carbon Capture Utilization and Storage (CCUS) can also be explored to complement India’s green hydrogen ambitions from a demand perspective.
Views are personal.
Krishnaveni Malladi is a Consultant (Hydrogen Technology) with the Sustainable Cities and Transport team at WRI India.